10:14 AM 5/28/2020 - Covid-19 and infected pork: Maybe, the answer is the INFECTED PORK: Hantavirus? Coronavirus? Co-infection? Other? Are the infected rats and pigs the silent asymptomatic carriers and the super-spreaders of Covid-19?

Covid-19 and infected pork: Maybe, the answer is the INFECTED PORK: Hantavirus? Coronavirus? Co-infection? Other? Are the infected rats and pigs the silent asymptomatic carriers and the super-spreaders of Covid-19?
________________________________________________________________
https://covid-19-review.blogspot.com/2020/05/1014-am-5282020-covid-19-and-infected.html
_________________________________________________________________
M.N.: Maybe, the answer is the INFECTED PORK: Hantavirus? Coronavirus? Co-infection? Other?ctajournal.biomedcentral.com/articles/10.11…
Covid-19 and infected pork - Google Search google.com/search?q=Covid…
Are the infected rats and pigs the silent asymptomatic carriers and the super-spreaders of Covid-19? - Google Search google.com/search?newwind…
_______________________________________________________________
- Review
- Open Access
- Published:
Is diet partly responsible for differences in COVID-19 death rates between and within countries?
Abstract
Reported COVID-19 deaths in Germany are relatively low as compared to many European countries. Among the several explanations proposed, an early and large testing of the population was put forward. Most current debates on COVID-19 focus on the differences among countries, but little attention has been given to regional differences and diet. The low-death rate European countries (e.g. Austria, Baltic States, Czech Republic, Finland, Norway, Poland, Slovakia) have used different quarantine and/or confinement times and methods and none have performed as many early tests as Germany. Among other factors that may be significant are the dietary habits. It seems that some foods largely used in these countries may reduce angiotensin-converting enzyme activity or are anti-oxidants. Among the many possible areas of research, it might be important to understand diet and angiotensin-converting enzyme-2 (ACE2) levels in populations with different COVID-19 death rates since dietary interventions may be of great benefit.
Introduction
A novel strain of human coronaviruses, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), named by the International Committee on Taxonomy of Viruses (ICTV) [1], has emerged and caused an infectious disease referred to as “coronavirus disease 2019” (COVID-19) by the World Health Organization (WHO) [2]. COVID-19 has aggressively spread across the globe and over 160,000 deaths have been reported. However, there appears to be high- and low-death rate countries.
After the outbreak in China, COVID-19 has also affected Europe after becoming a pandemic. Interestingly, there is large variability across European countries in both incidence and mortality, and most current debates on COVID-19 focus on the differences among countries. German fatalities are strikingly low as compared to many European countries. Among the several explanations proposed, an early and large testing of the population was put forward [3].
However, little attention has been given to regional differences and diet [4].
Biases to be considered
According to the Johns Hopkins coronavirus resource center (https://coronavirus.jhu.edu), one of the most important ways of measuring the burden of COVID-19 is mortality. However, death rates are assessed differently between countries and there are many biases that are almost impossible to assess. Differences in the mortality rates depend on the characteristics of the health care system, the reporting method, whether or not deaths outside the hospital have been counted and other factors, many of which remain unknown. Countries throughout the world have reported very different case fatality ratios—the number of deaths divided by the number of confirmed cases—but these numbers cannot be compared at all due to biases. On the other hand, for many countries, the methodology reporting death rates in the different regions is standardized across the country.
European data on death rates per million inhabitants
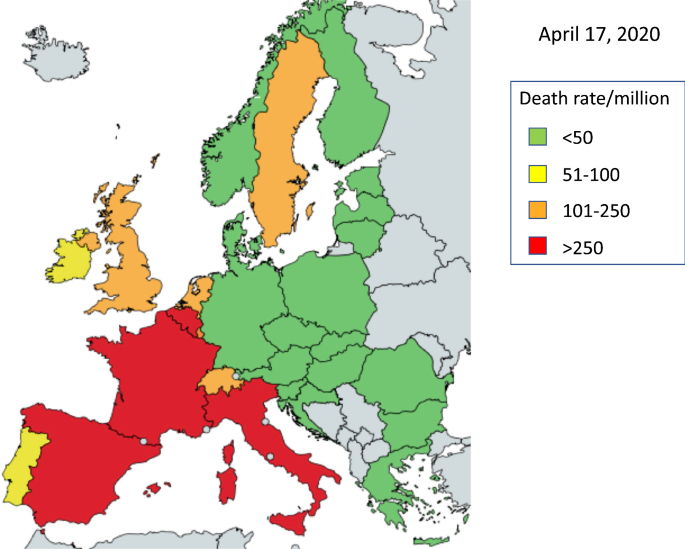
We used the Johns Hopkins coronavirus resource center to assess death rates at the national level (https://coronavirus.jhu.edu). The current death rate per million people in Europe shows different trends. Germany has a low death rate, but Austria, the Czech Republic, Poland, Slovakia, the Baltic States and Finland have similar or lower rates. On the other hand, Belgium, France, Italy, Spain and the UK have higher rates (Fig. 1).
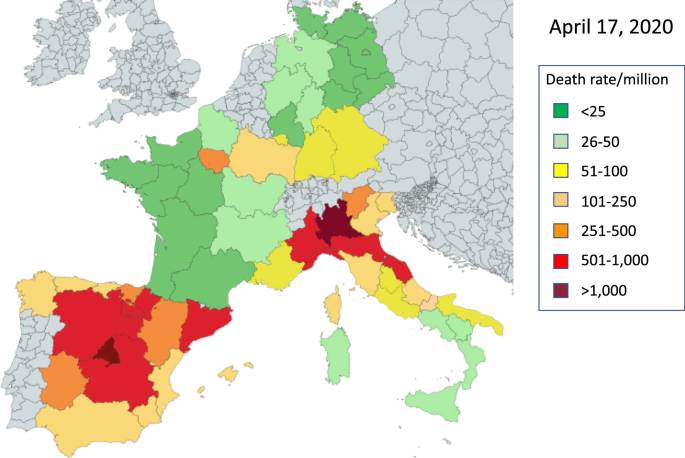
Large differences exist when assessing death rates within a country. In Germany, Bavaria started the earliest tests but was and still is the most affected region (Fig. 2). Death rates per million range from 8 in Mecklenburg-Vorpommern to 87 in Bavaria.
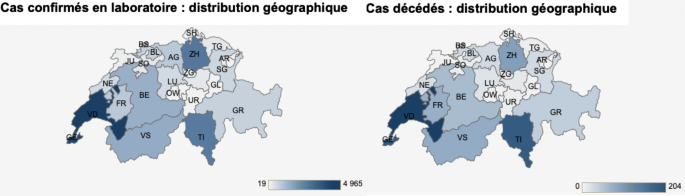
In Switzerland, the French and Italian speaking cantons have a far higher death rate than the German-speaking ones (Fig. 3) (Office fédéral de la santé publique, Switzerland, https://www.bag.admin.ch/bag/fr/home.html).
In high-rate countries such as Spain, large variations also exist within the country, but the numbers range from 115 in Murcia to over 1000 in Madrid.
Is diet partly involved in different death rates between countries?
Most diseases exhibit large geographical variations which frequently remain unexplained despite abundant research [5]. COVID-19 will not be an exception. Though the more relevant factors are likely to be seasonal variations, immunity, cross-immunity, intensity, timing of measures [6], type, onset, duration and measures of protection, other factors like environment or nutrition should not be overlooked. Obesity, a risk factor of mortality in COVID-19, suggests the importance of nutrition [7].
The “low-rate” European countries have used different quarantine and/or confinement times and methods and none have performed as many early tests as Germany. Thus, although the German testing approach is very important [3], other factors may also be significant.
Immunity in COVID-19 and ageing
Although there are large differences between countries in death rates, the age-dependent severity of COVID-19 is similar between Asian, European and American countries. The rate of deaths is increased in the older population. Globally, there are risk factors for death including obesity and type 2 diabetes.
A strong relationship between hyperglycemia, impaired insulin pathway, and cardiovascular disease in type 2 diabetes is linked to oxidative stress and inflammation [8]. Lipid metabolism has an important role to play in obesity, diabetes and its multi-morbidities, and the ageing process [9]. Dietary fatty acids have a significant role in immune responses [10].
Many foods have an antioxidant activity [11,12,13]. Resveratrol, present in many foods [14], is an inhibitor of MERS-Coronavirus infection [15].
Angiotensin-converting enzyme 2 (ACE-2)
The angiotensin-converting enzyme (ACE2) has multiple physiological roles: a negative regulator of the renin-angiotensin system, facilitator of amino acid transport, and the SARS-CoV and SARS-CoV-2 receptor [16]. ACE converts angiotensin I to angiotensin II but ACE2 catalyses the conversion of angiotensin II to angiotensin and is also the main entry point for coronavirus 2 into cells.
Differences between countries in ACE have been associated with genetic patterns. The ACE D allele increased risk of vasculitis [17] or hypertension [18]. The ACE I/D polymorphism is involved in the onset of type 2 diabetes [19] and might be associated with susceptibility to peripheral vascular diseases in the Asian population [20].
However, dietary patterns have a strong effect on ACE levels. A high-saturated fat diet increases ACE [21]. Many foods have an ACE-inhibitory activity [22,23,24]. Anti-oxidant activities and ACE inhibition have been largely found in many foods [25]. Moreover, ACE levels in blood are highly and rapidly sensitive to food intake [26].
Identifying whether countries with high or low ACE activity have different death rates would be of great interest in understanding the clinical importance of interventions. However, the available evidence, in particular from human studies, does not seem to support the hypothesis that inhibitors of ACE or renin-angiotensin–aldosterone (ACEI/ARB) drugs increase the ACE2 expression and the risk of COVID-19 [27]. This might suggest that changes in ACE expression (inhibition/stimulation) might not be as relevant as previously thought and other diet-related changes might be more (or equally) important.
Possible interactions between diet and COVID-19 death rate
Germany, Austria, Croatia, the Czech Republic, Poland, Slovakia, the Baltic States and German-speaking Swiss cantons exhibit lower COVID-19 mortality rates than France, Italy, Spain, and the French and Italian speaking Swiss cantons. Among many factors, diet differs considerably between these low- or high-mortality countries.
It appears that death rates in Germany are higher in the two Southern Regions as well as in Saarland than elsewhere. Baden-Wurttemberg and Saarland are in close contact with Alsace (France), and the higher infection rate may be due to the high cross-border traffic of the French. However, this was not the case for Rhineland-Palatinate (lower death rate), possibly because the East Region of France was contaminated later. In addition, Saarland is a special case as half of the deaths, unlike in the other German states, occurred in only a few long-term care facilities where a high number of people were infected in a short time and all deaths during the episode were attributed to Corona without autopsies being made. This potential French-based contamination does not apply for Bavaria (earliest German region to be contaminated and highest death rate). Diet differs within Germany, the southern states traditionally having a higher fat-rich diet. Diet is not normally distributed within country/region, which can be an additional argument in favour of the uneven distribution of mortality.
Nutrition may therefore play a role in the immune defense against COVID-19 and may explain some of the differences seen in COVID-19 across Europe. It will be needed to test dietary differences between low and high-rate countries. Foods with potent antioxidant or anti ACE activity—like uncooked or fermented cabbage [28,29,30]—are largely consumed in low-death rate European countries, Korea and Taiwan, and might be considered in the low prevalence of deaths.
Although it is difficult to compare health systems and death reporting across European countries, Bulgaria, Greece and Romania have very low death rates. This might also be associated with diet since cabbage (Romania) and fermented milk (Bulgaria and Greece) are common foods. The latter food is a known ACE natural inhibitor [31]. Turkey, another apparently low-death rate country, also consumes a lot of cabbage and fermented milk products.
Another example may be the food supply chain. The increasing availability of foods from big retail is a revolutionary event that has impacted crops (favouring those that have the best ratio of effectiveness over costs of production) and health at a population-size level. In particular, such a change in food availability has altered alimentary habits—promoting sugar-enriched, vitamin-depauperated foods—and has become one of the causes of the obesity epidemic, especially among adolescents. These foods come from centralized farms in selected areas of the world that are distributed around the planet, elongating the supply chain of food. The impact of long supply chain of food on health is measurable by an increase in metabolic syndrome and insulin resistance [32]. Therefore, rural areas that are more prone to short supply food may have been able to better tolerate the COVID-19 pandemia, with a lower death toll. These considerations may be partly involved in lower death rates in Southern Italy compared to the Northern part.
Conclusions
Understanding the within and between country differences in COVID-19 will be of paramount importance in understanding COVID-19 risk and protective factors, and will eventually help to control the epidemics.
We acknowledge that many factors may play a role in the extension and severity of COVID-19, such as trained immunity of the population, early and fast education, rapid organization and adaptation of the hospitals and the public, preparedness for pandemics and public hygiene. Diet represents only one of the possible causes of the COVID-19 epidemic and its importance needs to be better assessed.
Availability of data and materials
Not applicable.
Abbreviations
- ACE:
- Angiotensin converting enzyme
- COVID-19:
- Coronavirus 19 disease
- MERS:
- Middle East Respiratory Syndrome
- SARS:
- Severe acute respiratory syndrome
- SARS-Cov-2:
- Severe acute respiratory syndrome coronavirus 2
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020. https://doi.org/10.1038/s41564-020-0695-z(Epub ahead of prin).
- 2.Bousquet J, Akdis C, Jutel M, Bachert C, Klimek L, Agache I, et al. Intranasal corticosteroids in allergic rhinitis in COVID-19 infected patients: an ARIA-EAACI statement. Allergy. 2020. https://doi.org/10.1111/all.14302.
- 3.Stafford N. Covid-19: Why Germany’s case fatality rate seems so low. BMJ. 2020;369:m1395.
- 4.Bousquet J, Czarlewski W, Blain H, Zuberbier T, Anto J. Rapid response: why Germany’s case fatality rate seems so low: is nutrition another possibility. BMJ. 2020. https://www.bmj.com/content/369/bmj.m1395/rr-12.
- 5.Sunyer J, Jarvis D, Pekkanen J, Chinn S, Janson C, Leynaert B, et al. Geographic variations in the effect of atopy on asthma in the European Community Respiratory Health Study. J Allergy Clin Immunol. 2004;114(5):1033–9.
- 6.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–8.
- 7.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020. https://doi.org/10.1002/oby.22831.
- 8.Guzik TJ, Cosentino F. Epigenetics and immunometabolism in diabetes and aging. Antioxid Redox Signal. 2018;29(3):257–74.
- 9.Miedema MD, Maziarz M, Biggs ML, Zieman SJ, Kizer JR, Ix JH, et al. Plasma-free fatty acids, fatty acid-binding protein 4, and mortality in older adults (from the Cardiovascular Health Study). Am J Cardiol. 2014;114(6):843–8.
- 10.Radzikowska U, Rinaldi AO, Celebi Sozener Z, Karaguzel D, Wojcik M, Cypryk K, et al. The influence of dietary fatty acids on immune responses. Nutrients. 2019;11(12):2990.
- 11.Jain S, Buttar HS, Chintameneni M, Kaur G. Prevention of cardiovascular diseases with anti-inflammatory and anti-oxidant nutraceuticals and herbal products: an overview of pre-clinical and clinical studies. Recent Pat Inflamm Allergy Drug Discov. 2018;12(2):145–57.
- 12.Razmpoosh E, Javadi M, Ejtahed HS, Mirmiran P. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2016;32(2):143–68.
- 13.Serino A, Salazar G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients. 2018;11(1):53.
- 14.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5):946.
- 15.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144.
- 16.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020;126:1456–74.
- 17.Turgut S, Turgut G, Atalay EO, Atalay A. Angiotensin-converting enzyme I/D polymorphism in Behcet’s disease. Med Princ Pract. 2005;14(4):213–6.
- 18.Di Pasquale P, Cannizzaro S, Paterna S. Does angiotensin-converting enzyme gene polymorphism affect blood pressure? Findings after 6 years of follow-up in healthy subjects. Eur J Heart Fail. 2004;6(1):11–6.
- 19.Muthumala A, Gable DR, Palmen J, Cooper JA, Stephens JW, Miller GJ, et al. Is the influence of variation in the ACE gene on the prospective risk of Type 2 diabetes in middle-aged men modified by obesity? Clin Sci. 2007;113(12):467–72.
- 20.Han C, Han XK, Liu FC, Huang JF. Ethnic differences in the association between angiotensin-converting enzyme gene insertion/deletion polymorphism and peripheral vascular disease: a meta-analysis. Chronic Dis Transl Med. 2017;3(4):230–41.
- 21.Schuler R, Osterhoff MA, Frahnow T, Seltmann AC, Busjahn A, Kabisch S, et al. High-saturated-fat diet increases circulating angiotensin-converting enzyme, which is enhanced by the rs4343 polymorphism defining persons at risk of nutrient-dependent increases of blood pressure. J Am Heart Assoc. 2017;6(1):e004465.
- 22.Iwaniak A, Minkiewicz P, Darewicz M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Comprehens Rev Food Sc Food safety. 2014;13(2):111–34.
- 23.Ganguly A, Sharma K, Majumder K. Food-derived bioactive peptides and their role in ameliorating hypertension and associated cardiovascular diseases. Adv Food Nutr Res. 2019;89:165–207.
- 24.Fan H, Liao W, Wu J. Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J Food Biochem. 2019;43(1):e12572.
- 25.Huang AF, Li H, Ke L, Yang C, Liu XY, Yang ZC, et al. Association of angiotensin-converting enzyme insertion/deletion polymorphism with susceptibility to systemic lupus erythematosus: a meta-analysis. Int J Rheum Dis. 2018;21(2):447–57.
- 26.Tejpal S, Sanghera N, Manoharan V, Planas-Iglesias J, Bastie CC, Klein-Seetharaman J. Angiotensin converting enzyme (ACE): a marker for personalized feedback on dieting. Nutrients. 2020;12(3):660.
- 27.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2008975.
- 28.Dang Y, Zhou T, Hao L, Cao J, Sun Y, Pan D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J Agric Food Chem. 2019;67(24):6757–64.
- 29.Gharehbeglou P, Jafari SM. Antioxidant components of brassica vegetables including turnip and the influence of processing and storage on their anti-oxidative properties. Curr Med Chem. 2019;26(24):4559–72.
- 30.Patra JK, Das G, Paramithiotis S, Shin HS. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front Microbiol. 2016;7:1493.
- 31.Ahtesh FB, Stojanovska L, Apostolopoulos V. Anti-hypertensive peptides released from milk proteins by probiotics. Maturitas. 2018;115:103–9.
- 32.Santulli G, Pascale V, Finelli R, Visco V, Giannotti R, Massari A, et al. We are what we eat: impact of food from short supply chain on metabolic syndrome. J Clin Med. 2019;8(12):2061.
Acknowledgements
_______________________________________________________________
____________________________________________________________













Comments
Post a Comment